Greenwich LifeSciences Phase 3 Trial Should Be Stopped Due To The Manipulation of the Phase 2 Data
- Greenwich LifeSciences (GLSI) is a pre-revenue biotech that has only one experimental drug in its pipeline, GP2, which is a vaccine to prevent the recurrence of breast cancer following surgery, currently in Phase 3 trials.
- All peer reviewed publications of the GP2 Phase 2 results show a lack of statistical significance, which suggests the vaccine is a failure.
- GLSI claims statistical significance in the Phase 2 trial only after data manipulation which they presented in an obscure poster presentation.
- GLSI’s poster has numerous red flags, such as false statements and the unexplained exclusion of patients from the data.
- Announced in mid-2022, the Phase 3 study struggles to enroll patients. We spoke with some of the study’s site contacts, and learned that 12 of the sites that have been actively recruiting for 7 months haven’t found a single willing participant for the trial. No site contact that we’ve interviewed has recruited anyone yet.
- With the Phase 3 study’s current design, we estimate the final vaccine data results won’t be finished for another 12-20 years.
- GLSI’s CEO, Snehal Patel, uses the company like it’s his personal piggybank for him and his family.
- Patel had the company buy a Covid vaccine from him, then did nothing with it.
- To pump the stock, Patel and another executive, Jaye Thompson, have made tiny insider buys that are much less than what they receive in salary from GLSI.
- Galena, which tried to develop a similar vaccine and was initially led by the same group of people, was a pump and dump scheme; we see GLSI as a similar insider enrichment scheme.
- We assign a $3 price target on GLSI.
Greenwich Lifesciences (GLSI) is testing a potential cancer vaccine called GP2 (also GLSI-100), currently in a Phase 3 trial, for women who previously had breast cancer surgery. GP2 is the only drug in its pipeline.
Other than the mandatory index funds, no managed funds or institutional investors own shares of GLSI – it’s all uninformed retail investors and insiders. GLSI also doesn’t have any collaborations with big pharma. There are good reasons for this. The main reason is GP2 has already failed its early trials. In order to justify conducting a Phase 3 trial, GLSI selectively removed patients from the Phase 2 trial data in order to get its p-value to an acceptable level. GLSI’s ad-hoc slicing and dicing of the data, which often lacks explanation, is unethical. There were numerous red flags from the poorly-done trial and data releases that we go over in this report.
Given the lack of clinical evidence of vaccine efficacy, we believe that the Phase 3 study is unfair for patients who have to be subjected to three years of injections and its side effects, right after two years of harsh treatments they went through for breast cancer. Because of the lack of participants in the trial, it will conservatively be 12+ years before the final Phase 3 trial data will be revealed.
We believe the Phase 3 trial is unethical and should be stopped. We also believe it is destined to be a failure.
Greenwich’s CEO Treats The Company Like His Personal Piggy Bank
Before we go into the details on GP2’s phase 2 trial data, let’s get an insight into what the insiders’ plans are for the company.
GLSI’s CEO, Senal Patel, gets the stock up by doing little, token, insider purchases. But those are a tiny amount compared to his compensation, as shown below:
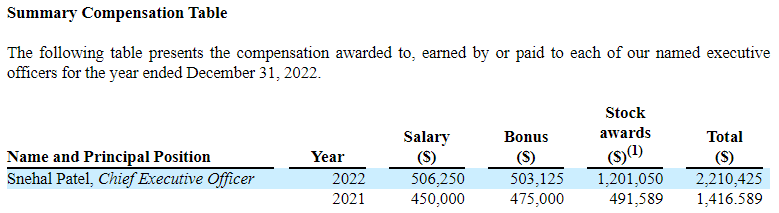
Source: GLSI 2022 10-K
We believe Patel’s compensation for 2022, a whopping $2.2M, is egregious for a CEO of a company that isn’t doing much, just waiting while the trial continues. His compensation is a lot more than the total amount of insider purchases he has done this year and last year. As the company sells stock with its ATM, it essentially pays back Patel for his insider purchases. The VP of Clinical Affairs, Jaye Thompson, also makes tiny insider buys, only 250 shares at a time.
On 12/15/20 GLSI announced it purchased an option for a pre-clinical covid vaccine candidate from a company called Westport Bio and GLSI agreed to conduct research on it. Westport Bio is Patel’s company and is registered at Patel’s home address. This is a clear conflict of interest. The cash went from GLSI straight into the CEO’s pocket for his covid vaccine that was never even made. GLSI never mentioned Westport Bio or its covid vaccine again after this PR.
Westport Bio’s address shown below:
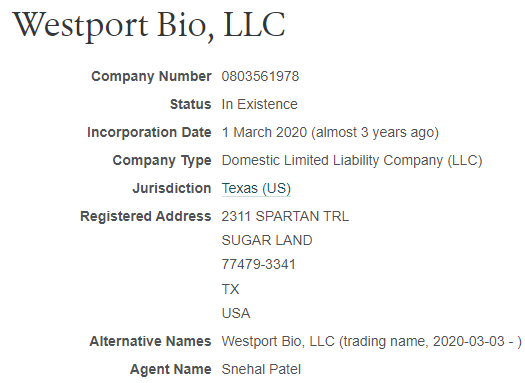
Source: opencorporates.com
Patel’s home address is exactly the same, as shown below:
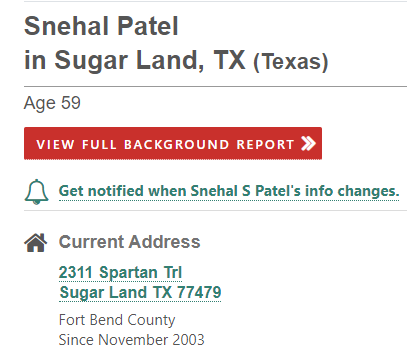
Source: fastpeoplesearch.com
It’s ridiculously high salaries and shenanigans like the above that the insiders will profit off of while the company messes around with a bogus Phase 3 trial that will likely go on for another 12-20 years.
Greenwich’s Claimed Statistical Significance of GP2 Effectiveness Is The Result of Phase 2 Data Manipulation
We believe GP2 failed its Phase 2 trial. The peer-reviewed results revealed the p-value was not high enough to justify doing a Phase 3 trial. Additionally, the Phase 2 trial was poorly done which makes the results untrustworthy.
The Phase 2 trial was originally done by Dr. George Peoples and his assistant, Dr. Elizabeth Mittendorf, in 2012 and 2013. They published an analysis in 2016 which had a median 34 months patient follow-up.
P-values for an estimated 5Y survival ranged from 0.43 for all patients in the study to 0.08 for the narrowest sub-group of HER2 positive patients that completed the primary GP2 treatment protocol before the recurrence of breast cancer (for per treatment (PT) patients). A p-value needs to be below 0.05 to show statistical significance. Therefore, this preliminary analysis did not show statistical significance.
The next peer-reviewed publication on the Phase 2 trial was published in April, 2020. It had a median follow-up of 41 months for all patients and 48 months for HER2 positive patients. Since it had a longer follow-up, its p-value for an estimated 5Y survival changed and ranged from 0.92 for all patients in the study to 0.052 for the narrowest sub-group of HER2 positive PT patients. Thus, this longer follow-up analysis still failed to prove statistical significance for GP2 treatment. It still wasn’t statistically significant since it was greater than 0.05. The publication even states:
“The primary analyses found no difference in 5-year overall disease-free survival (DFS) but possible benefit in subgroups.”
The final analysis of all patients completing 5 years of follow-up was never published in a peer reviewed journal. This makes us believe that the results were not good and the company decided not to publish it. Instead, GLSI put together an obscure poster, published on 12/9/20, with many red flags and question marks. Here are the serious issues we identified in this poster:
- Two GP2 patients were removed from the study, with no explanation why.
- It excluded patients that didn’t receive trastuzumab treatments after surgery.
- While the poster claims a statistical significant p-value for the narrowest sub-group, we believe that a peer reviewed statistical analysis would indicate an unacceptably high probability of a Type II error in rejecting the null hypothesis.
- It states that not all patients received booster shots, which differs from the peer reviewed publications that did not reveal this information, this questions the publications’ accuracy and integrity.
- The poster contradicts itself in regards to which patients took trastuzumab.
- The original study administrators and clinicians, including Dr. Peoples and Dr.Mittendorf, are not among the poster authors.
- Related to the above, only biased GLSI employees authored the poster, many, if not all, of whom aren’t qualified to publish complex trial data analysis.
Delving into each red flag:
1. Two GP2 treatment group patients were removed from the study, with no explanation why
To reach statistical significance, the poster reduced the GP2 treatment group by two patients, and didn’t explain why. It is possible that these two patients had cancer recurrence during the last stages of the clinical study, and that’s the unjustified reason for their removal, then it would no longer be 100%.
As shown in the 2020 peer-reviewed publication, there were 48 patients in the GP2 group, as shown below (red underline ours):
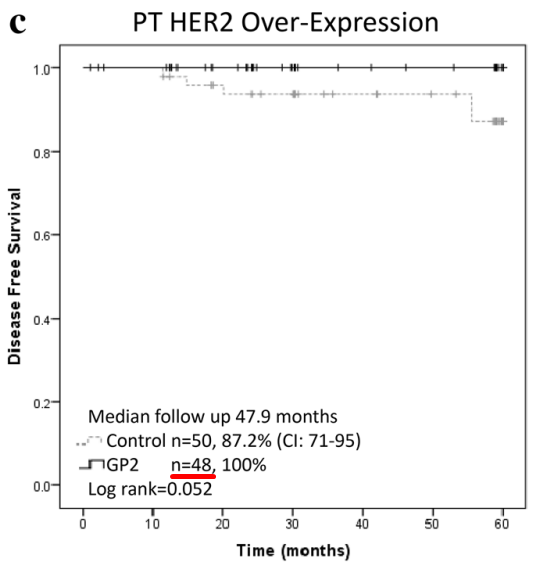
Source: Page 397, 2020 GP2 phase 2 trial peer-review publication
The publication defines a HER2 over-expressing tumor as IHC 3+. Then, looking at the poster, it only shows 46 patients in the GP2 HER2 3+ group (red underline ours):

Source: GP2 phase 2 trial poster
The number of patients in the placebo (or control) arm was the same in the poster and the publication: 50.
2. It excluded patients that didn’t receive trastuzumab treatments after surgery
To further decrease the p-value, patients who didn’t receive trastuzumab in the study were excluded from the data. The reason for this, according to the post-hoc analysis in the poster, is “GP2 is synergistic with trastuzumab”. However, this statement is misleading because this is based on post-hoc analysis, there is no solid evidence to verify it. In the 2020 publication it states: “GP2 may have synergistic clinical efficacy when combined with trastuzumab.”
Notice the word “may” which is absent in the statement on the poster. This hypothesis came from preclinical work by Dr. Mittendorf published in 2006.
3. While the poster claims a statistical significant p-value for the narrowest sub-group, we believe that a peer reviewed statistical analysis would indicate an unacceptably high probability of a Type II error in rejecting the null hypothesis.
Three patients from the HER2 3+ subgroup were removed by adding a PT (per treatment) requirement and an additional 2 patients were removed with no explanation. The probability of a Type II error is rapidly increasing with the decrease in the size of the sub-group. The original study protocol did not power the Phase 2 study for these narrow sub-group analyses.
Furthermore, as stated in this report from 2015 on the GP2 Phase 2 study:
“trastuzumab added to chemotherapy increased 10-year overall survival from 75.2% to 84% and 10-year DFS from 62.2% to 73.7%, Salazar said. “So the question becomes, if you add the vaccine to trastuzumab to prevent recurrence, does it improve survival even more?”
We believe it’s likely that the trastuzumab drug is what’s preventing recurrence, and GP2 isn’t doing anything.
It also states:
“The numbers are too small to draw a conclusion without knowing specifics about the HER2+ patients who had a recurrence and those who did not, and whether those arms were balanced in terms of tumor size and nodal status,” said Edith A. Perez, M.D., deputy director at large at the Mayo Clinic Cancer Center in Jacksonville, Fla. “
These reasons increase the risk of a Type II error.
4. It states that not all patients received booster shots, which differs from the peer reviewed publications that did not reveal this information, this questions the publications’ integrity.
On the poster it states (red underline ours):

Source: GP2 phase 2 trial poster
Boosters are a big part of this trial. This statement undermines the trial and the publications. The publications claim that all patients received the boosters. If indeed not all patients received boosters, then the publications hid this information and therefore should be amended. In addition, we don’t know which patients in which group received the boosters and which didn’t. So it is a mess and adds a question mark to the study quality overall.
5. The poster contradicts itself in regards to which patients took trastuzumab
The following evidence illustrates the poster’s sloppiness and incorrect information by clearly contradicting itself. In the RESULTS section of the poster, it states that all 96 HER2 3+ patients took trastuzumab after their breast cancer surgery, shown below (underline ours):

Source: GP2 phase 2 trial poster
Then in Figure 1 of the poster it states that the following HER2 3+ subjects completed PIS (Primary Immunization Series) following trastuzumab.
But this is contradicted at the bottom of that same Figure 1, which states that only 89% received trastuzumab, shown below (underline ours):
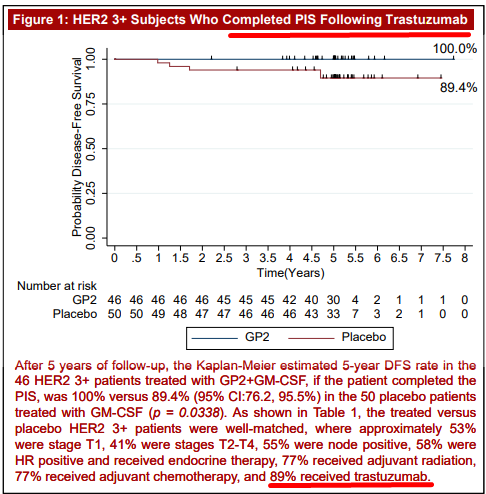
Source: GP2 phase 2 trial poster
6. The original study administrators and clinicians, including Dr. Peoples and Dr. Mittendorf, are not among the poster authors.
It takes a lot of work and dedication to manage and carry-out a drug trial. When a new publication or poster comes out, it’s natural for the conductors of the trial to want to get credit for their work, and they should. That’s why it’s strange that neither Dr. Peoples nor Dr. Mittendorf are mentioned on this poster. Why is that when all of the info in the poster comes from their clinical research? We believe the plausible explanation is that the clinicians did not support the highly manipulative data analysis presented in the poster and did not want to put their names behind it.
7. Related to the above, only biased GLSI employees authored the poster, many, if not all of whom, aren’t qualified to publish complex trial data analysis.
Instead of the original trial administrators, a bunch of people who had almost nothing to do with the trial are mentioned, such as the CEO’s daughter, Marisa Patel.
The Phase II Trial Was Done Poorly, And Should be Redone
Our analysis of clinical data presented above indicates that the GP2 Phase 2 trial data are inconsistently presented, and therefore shouldn’t be relied upon. In addition, there were some issues with the conduct of the clinical trial itself, such as:
- The trial conductor mixed the patients with patients in another similar trial he was doing.
- The circulating tumor cell (CTC) assays weren’t performed.
- The data wasn’t all transferred over because Dr. Peoples demanded a $500K payment which GLSI declined to pay.
- Not all patients received the booster treatment and it is unclear who did and did not.
The GP2 phase 2 studies began when the Henry M. Jackson Foundation (HJF) entered two License Agreements with Norwell (now GLSI), regarding GP2. The agreements stipulated that Norwell would sponsor an ongoing clinical trial of GP2 being conducted by Dr. George Peoples. But things went bad quickly from research misconduct, and HJF sued Norwell. Details on the lawsuit can be found here.
Both parties agreed upon a flat fee of $3M to cover 180-300 patients enrolled in the GP2 study as necessary to reach statistical significance.
Norwell applied to a Texas state fund, the Texas Emerging Technology Fund (ETF), for a $2.5M grant to conduct the trial.
The grant was initially declined. Primarily because Dr. Peoples was actively researching the two other parts of the protein similar to GP2: AE37 and E75. This is a blatant conflict of interest because Peoples was also getting paid by the other two companies.
ETF later said it would agree to the $2.5M grant if Norwell distanced itself from Dr. Peoples and for Peoples to transfer over the trial data and IND. However, Peoples refused to hand it over unless Norwell paid him $500K. Norwell declined to pay Dr. Peoples the money.
Circulating Tumor Cell (CTC) assays were supposed to be performed on the patients in the trial. This wasn’t done.
Finally, we addressed the confusing information about boosters in the section above.
With The Phase 3 Study’s Current Design, We Estimate The Final Results Won’t Come For At Least Another 12 Years
On GLSI’s website it states the trial design is to enroll about 500 patients in the trial. We estimate that to finally report the results of all the patients needed for the trial, it will take over 12 years. It probably will even take longer.
Let’s say out of the 23 recruiting sites, they are able to get one patient every six months on average. We estimate this slow recruitment rate because, as we go into more detail later, we recently spoke with site contacts who said they haven’t been able to recruit a single patient yet.
This comes out to 46 patients per year across all 23 sites. Then let’s assume that 4 of them drop out and don’t continue for the full 3 years of treatment. That totals 42 patients per year. This means it would take 500/42 = 11.9 years to get the number of patients GLSI needs for the trial design. Then there’s about a five-year follow-up after the first dose of treatment. That would total over 16.9 years to get the final results of the phase 3 trial. And that doesn’t even include an extra year to apply for FDA approval. Even if they recruit patients faster than one every six months per site, and/or add more sites, it would still take well over 12 years to get the final data readout.
During all of these upcoming years the CEO and other insiders will enrich themselves with investors’ money.
On 8/11/22, GLSI announced the activation of clinical sites, and that “multiple sites have begun the screening and enrolling process.” Clinicaltrials.gov shows 23 sites currently recruiting.
On 3/13-3/15 we spoke with the lead contacts of some of the sites, and they revealed that over the 12 sites they cover, which have been actively recruiting since September 2022, they haven’t been able to recruit a single participant yet.
One of the reasons for this, is because HER2+ patients only represent 15-20% of breast cancer patients. This drastically lowers the eligible participants for the study. It also will decrease potential revenue If GP2 ever gets approved. Another reason is it isn’t worth it for most eligible patients to go through the trial.
It Is Not Worth It For Breast Cancer Patients To Participate In This Trial
In the words of one of the site contacts we interviewed:
“Here’s the thing that we’ve been up against, the patients who get their surgery and neo-adjuvant treatment, from the time of diagnosis to the time they’re done, is about a year and a half or close to 2 years.
So the issue is, they are done a lot of times. The patients say they don’t want any more. For all intensive purposes they are cured of their cancer, and they sometimes don’t want to go beyond that. So this trial, being an adjuvant treatment going beyond what they normally get, it’s essentially another 3 years.”
The patients aren’t getting paid anything or having their travel expenses reimbursed. They have no incentive to join this exploratory trial of a vaccine that probably doesn’t work.
The side effects of the therapy are unpleasant, as shown in the study description:
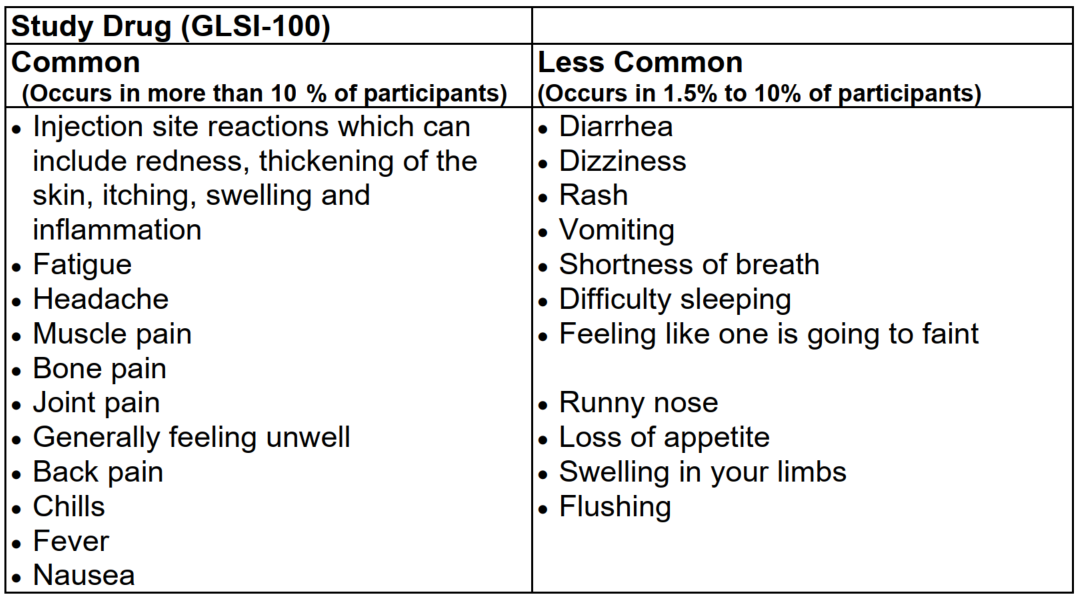
Source: GLSI-100 Phase 3 Trial description from Trial Site Contact
Furthermore, even if the patients believe the vaccine has potential, there is a chance that they would get the placebo. If they get the placebo then even if GP2 ends up working, they would have to go through another 3 years of treatment after that once it gets FDA approval, which would likely take another 15-20 years.
Another possible concern with this is what if trastuzumab is no longer the standard of care for breast cancer 15 years from now? The GP2 trial is done now to only apply to patients taking trastuzumab. The FDA may require GLSI to do a new study for it to be used with the new standard of care drug.
Other Companies That Tried To Develop From The Same HER2 Protein Failed
Dr. George Peoples was the Chief of Oncology of the US Army’s hospital. He was developing vaccines for breast cancer from a protein, HER2, which is associated with recurrence and low survival rates in breast cancer. He cut the HER2 protein into three different parts to essentially create three different vaccines. Each part went to a different company, which was Galena Pharma (now SELLAS, symbol SLS), Antigen Express, and Norwell (which changed its name to now Greenwich LifeSciences (GLSI)).
Galena’s vaccine was called NeuVax, as shown in this article from 1/5/12 titled: Galena Biopharma Initiates Patient Enrollment in NeuVax Phase 3 PRESENT Trial to Prevent Breast Cancer Recurrence.
NeuVax failed, and Galena ended up becoming a long running scam and stock promotion. Its CEO, Mark Ahn, settled with the SEC for stock promotion with Galena. He later did prison time for insider trading.
Similar to Galena, we believe GLSI is nothing but an enrichment scheme for the CEO and his family.
Just like NeuVax did, GP2 likely will fail as well. This study from 1999 showed that GP2 binds poorly to tumor cells. The scientists tried mutating the residue but that still didn’t improve its binding ability. From the abstract of the study:
In particular, GP2 (HER-2/neu residues 654-662) binds very poorly even though it is predicted to bind well based upon the presence of the correct HLA-A2.1 peptide-binding motif. Altering the anchor residues to those most favored by HLA-A2.1 did not significantly improve binding affinity.
Conclusion
We question the quality and professional judgment of GLSI’s management. Management designed and is conducting a bad trial both for patients and investors. The Phase 3 trial will drag on for well over a decade, and its chances for success are minimal due to the poor results of the Phase 2 study. This seems to be an unethical study to subject patients to. Similarly, investors have to fully realize their ROI prospects given the very long duration of the study, low probability of success, and only a small percentage of breast cancer patients are eligible due to the limiting requirements for patients in the study. 12-20 years of a Phase 3 study is good only for GLSI’s CEO and his management team, who are using GLSI as a vehicle to enrich themselves.
Because of the numerous red flags with the clinical data and protocols of the Phase 2 trial of GP2 and the poorly designed Phase 3 trial, we believe the drug is destined for failure. We have a $3 price target on GLSI.




